By Business Insider Reporter
The launch of the East African Community (EAC) Medicines Registration Harmonisation (MRH) Project marks a major turning point in how medicines are approved, supplied and accessed across the region, with far-reaching implications for public health systems, pharmaceutical markets and patients.
The initiative, spearheaded by the NEPAD Agency under the African Medicines Regulatory Harmonization (AMRH) Programme, brings together a powerful coalition of partners including the World Health Organization (WHO), the Bill & Melinda Gates Foundation, the World Bank, the UK Department for International Development (DfID), and the Clinton Health Access Initiative (CHAI). Its core objective is to align and streamline medicines registration processes across EAC member states, replacing fragmented national systems with a coordinated regional approach.
At its heart, the MRH Project seeks to shorten the time it takes for essential medicines to reach patients. Under the current system, pharmaceutical companies are often required to submit separate applications to each national medicines regulatory authority, a process that is costly, time-consuming and prone to delays. Harmonisation will allow a single scientific assessment to be recognised across multiple countries, significantly accelerating access to quality-assured medicines for priority diseases such as malaria, HIV/AIDS, tuberculosis and non-communicable diseases.
For patients, the impact could be profound. Faster approvals mean earlier access to life-saving treatments, reduced medicine shortages and improved confidence in the safety and effectiveness of drugs on the market. For governments, harmonised regulation promises better oversight, reduced duplication of effort and more efficient use of limited regulatory expertise and budgets.
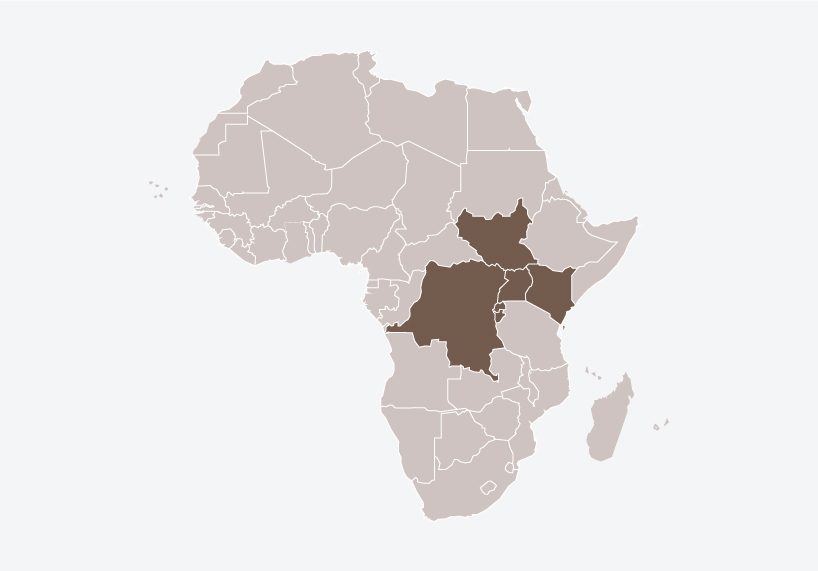
The project also addresses long-standing structural challenges facing Africa’s pharmaceutical sector. Many countries in the region struggle with weak or outdated medicines legislation, limited laboratory infrastructure and shortages of trained regulatory professionals.
By pooling technical capacity and aligning standards, the MRH Project strengthens regulatory systems while reducing dependence on external approvals from Europe or North America.
Importantly, the initiative is expected to stimulate local pharmaceutical manufacturing. Predictable and transparent regulatory pathways lower barriers to entry for regional manufacturers, making it more attractive to invest in production facilities within East Africa. This could help reduce reliance on imported medicines, improve supply chain resilience and support industrialisation and job creation.
Development partners see the MRH Project as a critical building block for Africa’s broader health security agenda. Harmonised regulation not only improves routine access to medicines but also enhances the region’s ability to respond to public health emergencies, such as pandemics, by enabling rapid approval and distribution of vaccines and therapeutics.
The EAC MRH Project aligns with continental efforts under the African Union to strengthen health sovereignty, including the establishment of the African Medicines Agency (AMA). Together, these initiatives signal a shift towards African-led solutions to Africa’s health challenges. As East Africa moves to operationalise the MRH framework, its success could serve as a model for other regions on the continent – demonstrating how regulatory cooperation can translate into healthier populations, stronger health systems and more inclusive economic growth.